Calorimetry is the measurement of specific heat capacity.
Specific Heat Capacity is the amount of energy required to raise the temperature of 1 gram of a substance by 1 degrees celsius.
The specific heat capacity of water is 1 calorie per grams degree celsius.
and 1 cal is equivalent to 4.184 Joules.
Temperature and heat flow can be understood as the movement of molecules.
Whew, that was confusing.
By the way, calories are different from Calories. 1 Calorie is 1000 calories (cal).
These are some calorimetry problems (with their solutions) that we worked on.
Hmm, what else...?
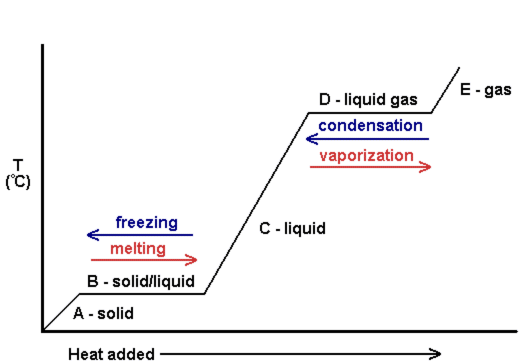
During phase changes, energy is not required.
See how the line is straight in the following graph?

No comments:
Post a Comment